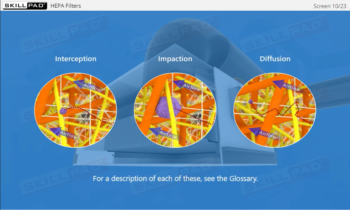
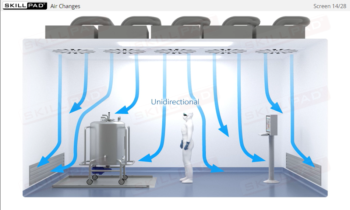
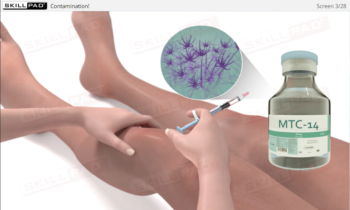
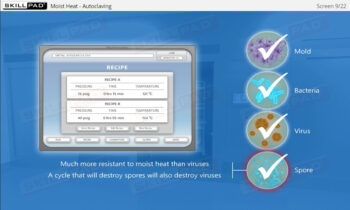
Aseptic Processing: Gowning
Describes the different gowning requirements typically used in aseptic manufacturing facilities to prevent contamination of products. It emphasizes the criticality of following correct gowning procedures for different cleanroom classes using ISO 5/6, ISO 7 and ISO 8 as examples.
Part of Annex 1 training requirements.
Duration: 30 Mins
Description
Learning Objectives:
|
Module Features:
Animations |
|---|