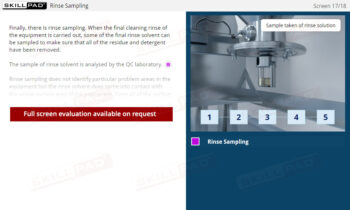
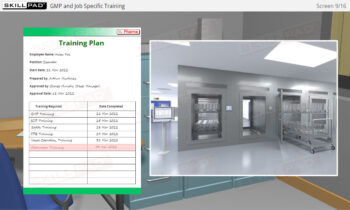
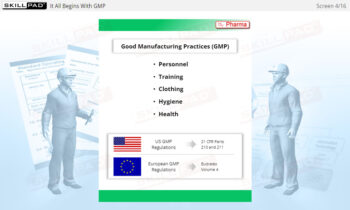
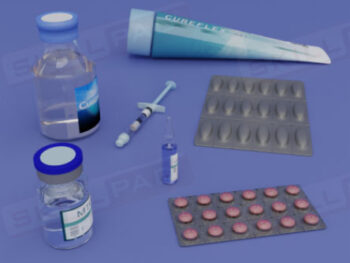
Buildings & Facilities
This Module is aimed at personnel who require an overview of GMP design requirements for a manufacturing facility, including product flow, environmental controls, water supply, cleaning and sanitation, and maintenance.
Duration: 30 Mins
Description
Learning Objectives:
|
Module Features:
Animations |
|---|
Keywords
| Construction Dead Spaces Design Humidity HVAC |
Maintenance Overcrowding Product Flow Sanitation Sensitizing Materials |
|---|