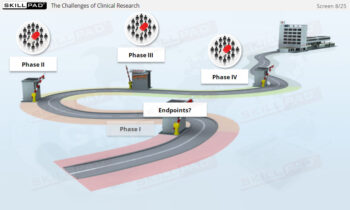
New Drug Development and Clinical Trials
Describes the most important characteristics of drug products and explains why the development and testing of new drug products must be regulated. It provides an overview of the drug development process and the various phases of clinical trials. It also introduces the concept of Good Clinical Practice (GCP).
Duration: 30 Mins
Description
Learning Objectives:
|
Module Features:
Animations |
|---|