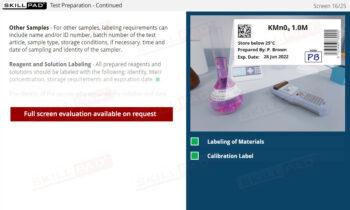
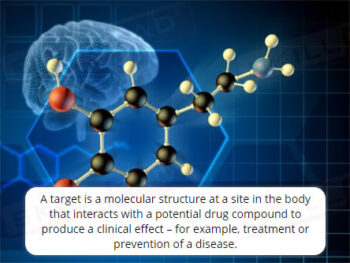
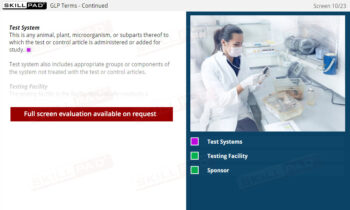
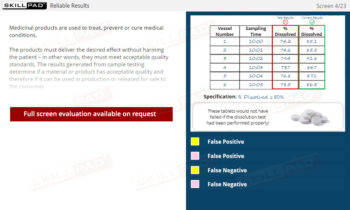
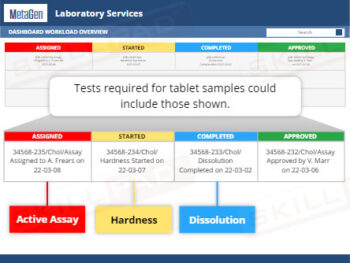
Out of Specification and Atypical Results
This Module is aimed at personnel who require an overview of ‘Out Of Specification’ (OOS) and Atypical Results in the analytical laboratory, including how they are investigated, and how they can be prevented.
Duration: 30 Mins
Description
Learning Objectives:
|
Module Features:
Animations |
|---|
Keywords
| Assignable Cause Atypical Result Barr Decision EMA FDA |
Laboratory Error Non-Assignable Cause OOS Investigation Out of Specification (OOS) Result Resampling |
|---|