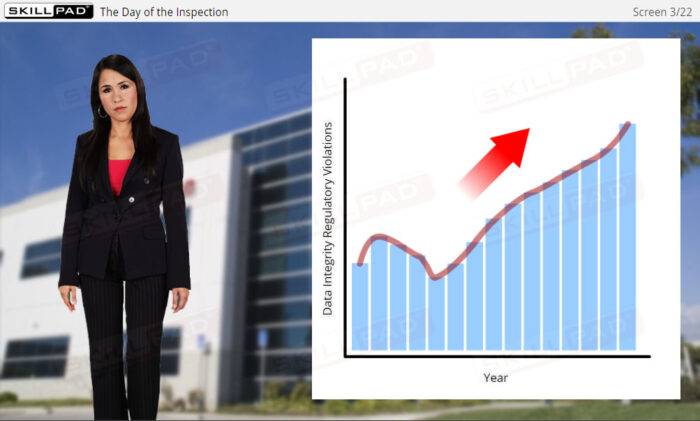
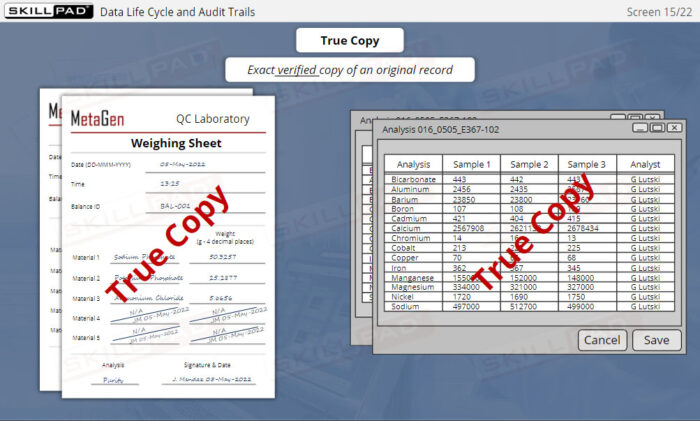
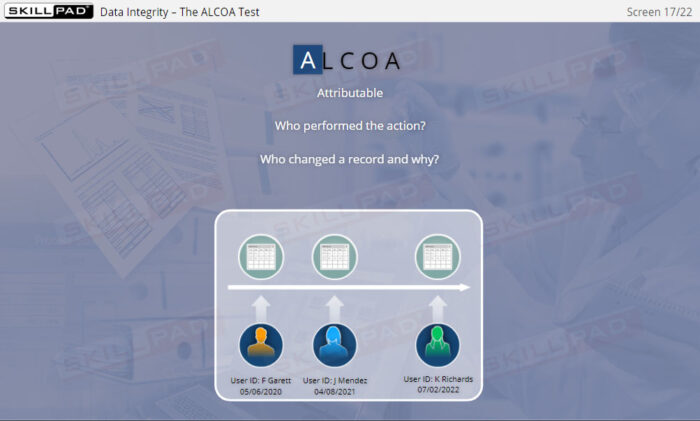
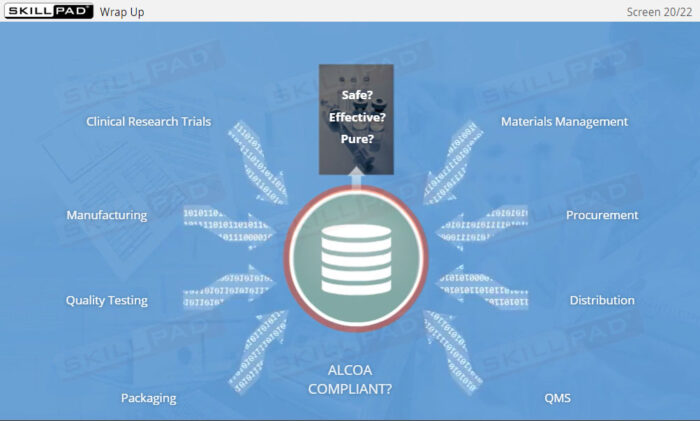
Data Integrity for GxP Regulated Industries
This Module is an overview of data integrity in regulated industries and its essential role in assuring product quality. Key data integrity concepts in GxP environments are explored along with regulatory requirements and expectations. The critical role of personal responsibility in maintaining data integrity compliance is also covered.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Description
Learning Objectives:
Learner Benefits:
|
Module Features:
Animations |
|---|