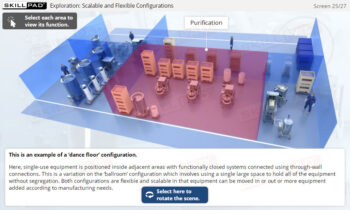
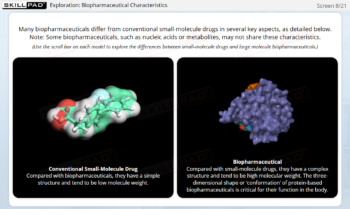
Essentials of Biopharmaceutical Manufacturing
A practical overview of how biopharmaceuticals are manufactured—from cell thawing and culture in upstream processing, through separation, purification, and formulation in downstream processing, to fill-finish and packaging. The module emphasizes the importance of contamination control and highlights the critical role of operators in ensuring product quality, regulatory compliance, and patient safety.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
- Understand the key steps in biopharmaceutical manufacturing, from cell thawing and culture to purification, formulation, and packaging.
- Learn how upstream and downstream processes contribute to product quality and patient safety.
- Recognize the importance of contamination control in ensuring product safety and quality.
- Gain insight into operator responsibilities and how their actions ensure compliance and product sterility.
- Become familiar with essential technologies used throughout biopharmaceutical manufacturing, including bioreactors, separation and purification systems, formulation equipment, and packaging processes.
Learning Objectives
- Describe how living cells are used to produce biopharmaceutical products.
- Identify the key steps in upstream processing, downstream processing, fill-finish, and packaging and serialization.
- Describe the purpose of each of the key steps in upstream processing, downstream processing, fill-finish, and packaging and serialization.
- Explain the importance of contamination control throughout biopharmaceutical manufacturing.
- Recognize the role of operators in maintaining product quality at each manufacturing step.
- Explain how operator actions contribute to product quality, regulatory compliance, and patient safety.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen