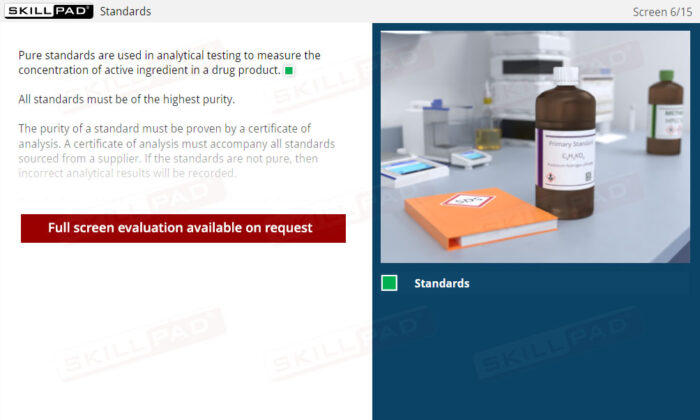
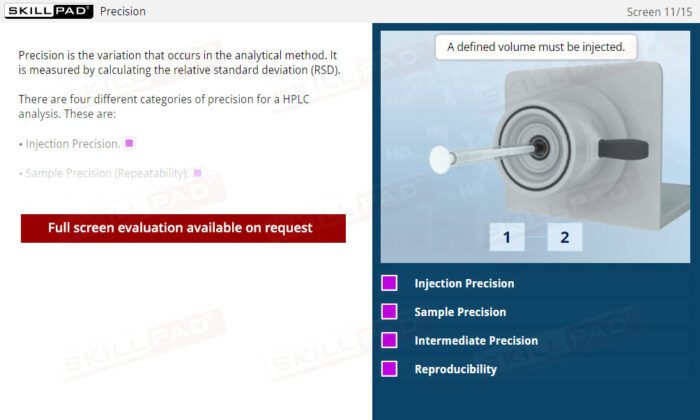
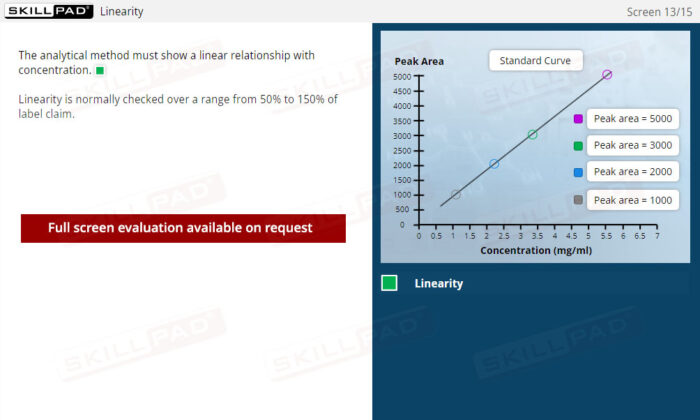
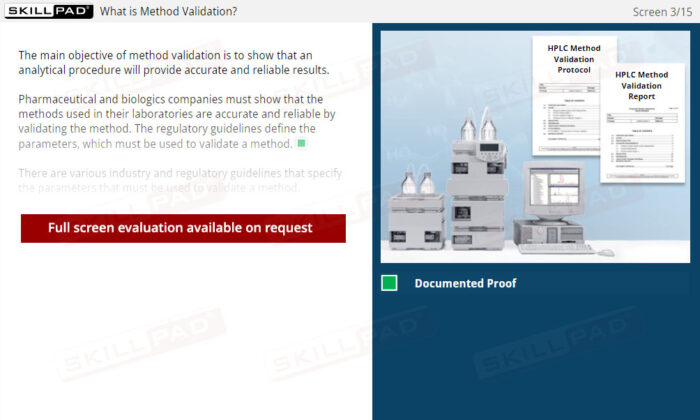
Method Validation Parameters
A comprehensive examination of the key parameters in analytical method validation, crucial for ensuring the accuracy and reliability of laboratory testing in pharmaceuticals and biologics. This module covers the essential aspects of method validation, including specificity, accuracy, precision, and sensitivity. It explores the differentiation between active ingredient and impurity analyses, explains critical concepts like Limit of Detection (LOD) and Limit of Quantitation (LOQ), and emphasizes adherence to regulatory standards for method validation as outlined by FDA, EMA, and ICH.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Clear Understanding of Validation Purpose: Develop a foundational understanding of why method validation is essential in ensuring laboratory test accuracy and regulatory compliance.
- Insight into Specific Analytical Techniques: Gain knowledge on Category 1 and Category 2 analyses, with detailed insights into standards used for measuring active ingredients and impurities.
- Enhanced Knowledge of Key Analytical Parameters: Learn critical definitions and applications of specificity, accuracy, and precision, and understand their roles in method validation.
- Awareness of Stability Testing Requirements: Understand the importance of sample stability during testing, ensuring accurate and reliable results under standard laboratory conditions.
- Mastery of Detection Limits in HPLC: Grasp the significance of the Limit of Detection and Limit of Quantitation, key indicators of an analytical method’s sensitivity and reliability in detecting and measuring impurities.
Learning Objectives
- Explain the purpose of analytical method validation.
- Explain the difference between Category 1 and Category 2 analyses.
- Define ‘Specificity’ as it relates to HPLC analysis.
- Explain the difference between the terms ‘Accuracy’ and ‘Precision’.
- Describe how stress testing is done as part of method validation.
- Define the term ‘Limit of Detection’.
- Define the term ‘Limit of Quantification’.
Keywords
- Accuracy
- Analytical Method Validation
- Biologics Methodology
- EMA Standards
- HPLC Analysis
- ICH Regulations
- Limit of Detection (LOD)
- Limit of Quantitation (LOQ)
- Pharmaceutical Testing
- Stress Testing
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen