Moist Heat Sterilization
An introduction to the principles and practices of moist heat sterilization using autoclaves. This module explains the science behind steam sterilization and its critical role in ensuring product sterility in pharmaceutical and biologics manufacturing. Learners will explore the design and operation of autoclaves, including key process parameters such as temperature, pressure, and sterilization time. The module also covers load preparation, sterilization stages, and troubleshooting deviations to maintain compliance with GMP standards.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
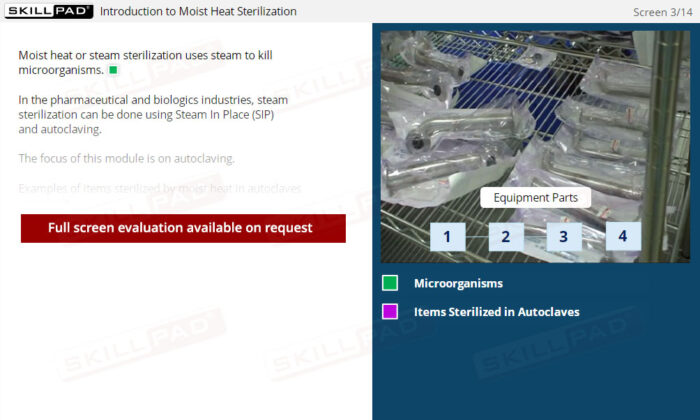
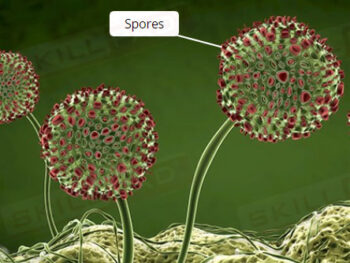
- Foundational Knowledge of Moist Heat Sterilization: Learn how steam sterilization eliminates microorganisms, the science behind its efficacy, and its applications in pharmaceutical and biologics manufacturing.
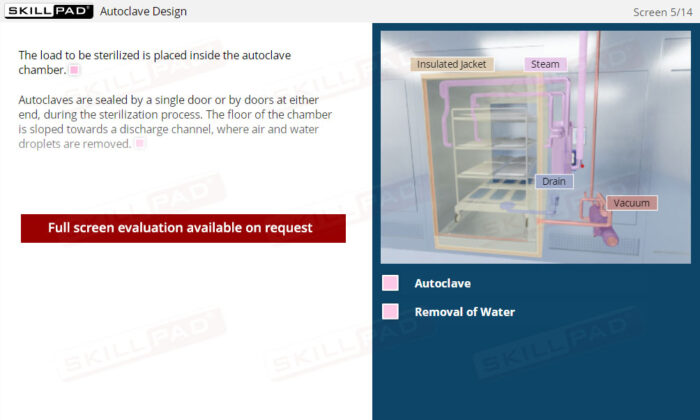
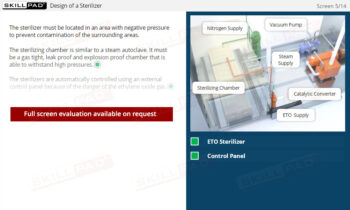
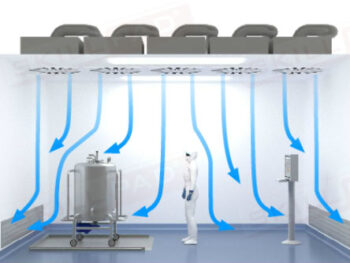
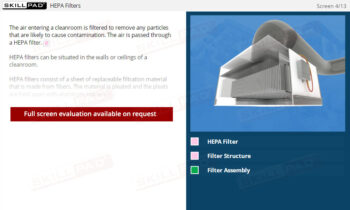
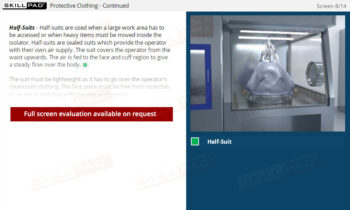
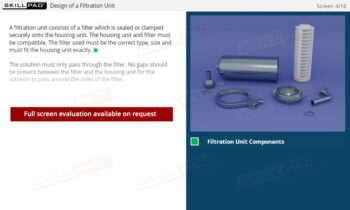
- Learn About Autoclave Operations: Gain foundational knowledge of autoclave design and operation, including key components, air removal methods, and steam penetration techniques essential for effective sterilization.
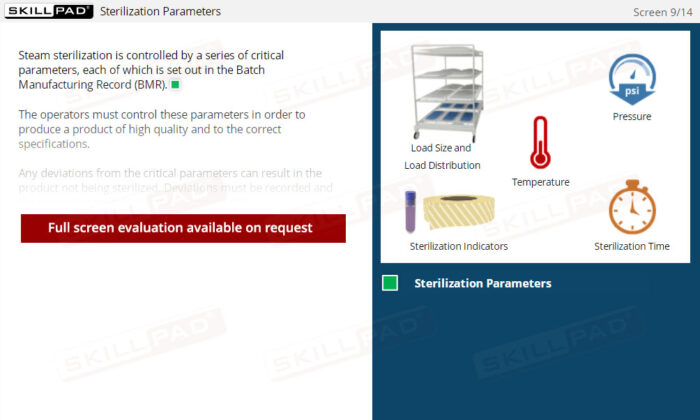
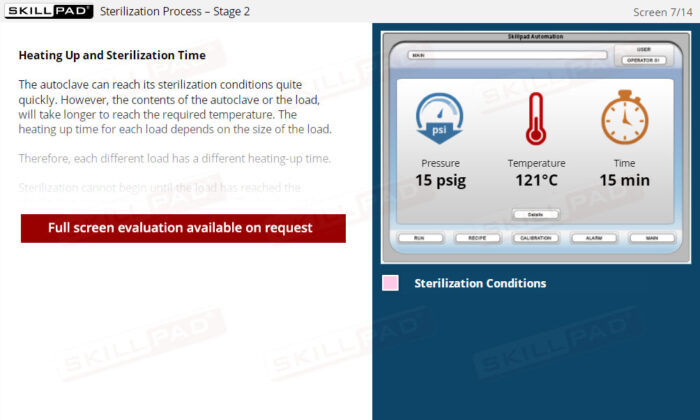
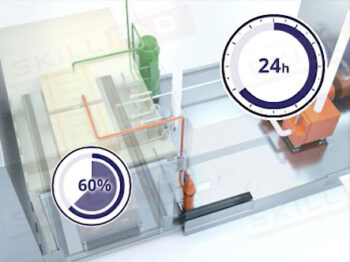
- Understand Critical Process Parameters: Understand how to monitor and control critical parameters like temperature, pressure, and sterilization time to ensure compliance with GMP requirements and maintain product sterility.
- Learn Load Preparation Best Practices: Discover how to organize and manage loads, including size considerations and the use of sterilization indicators, to achieve consistent and reliable sterilization outcomes.
- Develop Problem-Solving Skills: Learn how to identify and resolve common autoclave-related issues, such as air entrapment or incorrect parameter settings, to safeguard product quality and patient safety.
Learning Objectives
- Explain what is meant by moist heat sterilization.
- Describe the uses of moist heat sterilization via autoclaving.
- List the conditions needed for moist heat sterilization via autoclaving.
- Describe the design of an autoclave.
- List the different stages of moist heat sterilization via autoclaving.
- Describe the critical parameters of moist heat sterilization via autoclaving.
Keywords
- Autoclave
- Autoclave Chamber
- Batch Manufacturing Record (BMR)
- Biologics
- Critical Parameters
- Deviation
- Glassware
- GMP Compliance
- Heating-up Time
- Load Distribution
- Load Preparation
- Load Size
- Moist Heat Sterilization
- Pharmaceutical Manufacturing
- Pressure
- Product Sterility
- Saturated Steam
- Steam In Place (SIP)
- Steam Penetration
- Sterilization Cycle
- Sterilization Indicators
- Sterilization Process
- Sterilization Time
- Temperature
- Tubing
- Validation
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen