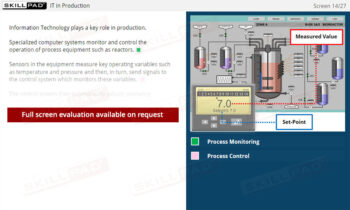
Overview of 21 CFR Part 11
Gain a clear understanding of the FDA’s 21 CFR Part 11 regulation, which governs the use of electronic records and signatures in regulated industries. Delve into the fundamental principles, terminology, and compliance requirements, including audit trails, electronic signatures, and data integrity. Learn about the distinctions between open and closed systems and the comprehensive security protocols necessary for ensuring regulatory compliance. This module provides a solid foundation for working confidently within FDA-regulated environments, emphasizing the importance of system validation, data protection, and documented procedures.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Understand 21 CFR Part 11 Compliance: Learn the key definitions, compliance criteria, and regulatory requirements to navigate and apply this regulation effectively.
- Manage Data Integrity in Electronic Records: Explore best practices for maintaining electronic records and signatures, ensuring data integrity and proper audit trails in line with FDA standards.
- Implement System Security and Compliance Measures: Learn the differences between open and closed systems and how security controls protect data from unauthorized access and regulatory breaches.
- Apply Validation Processes and System Controls: Understand essential validation procedures and system controls to ensure the accuracy and reliability of electronic records.
Learning Objectives
- Explain the purpose of the 21 CFR Part 11 regulation.
- Define electronic records and electronic signatures.
- Explain what is meant by a hybrid system.
- Compare ‘Open’ and ‘Closed’ systems.
- List the Part 11 compliance requirements for Closed and Open Systems.
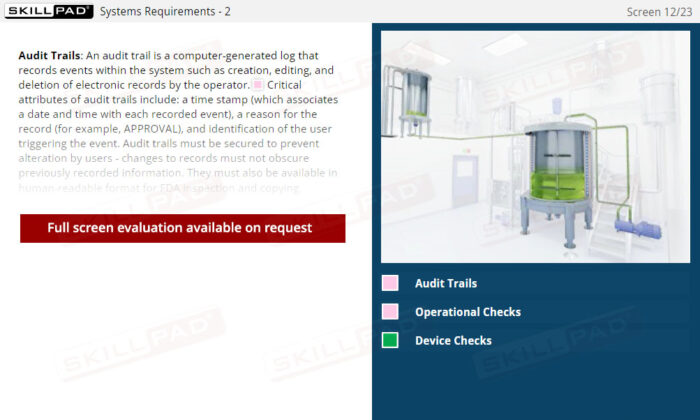
- Define audit trail and time stamp.
- Explain the purpose of operational, authority and device checks.
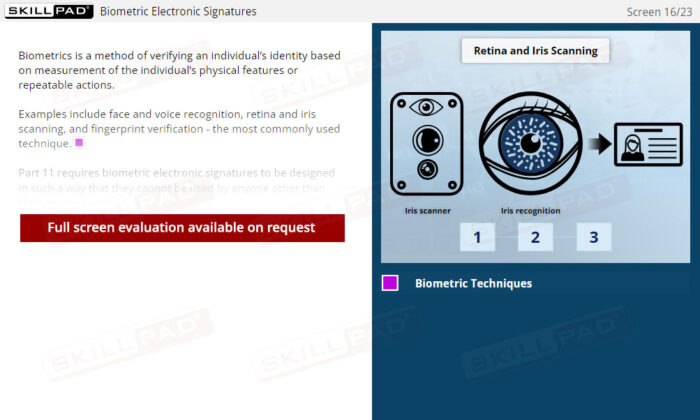
- Distinguish between biometric and non-biometric signatures and list their Part 11 requirements.
- Explain ‘linking’ of electronic signatures and records.
- Summarize the general action points required for achieving Part 11 compliance.
Keywords
- 21 CFR Part 11
- Access Controls
- Audit Trails
- Closed Systems
- Compliance
- Data Integrity
- Electronic Records
- Electronic Signatures
- FDA Regulations
- Hybrid Systems
- Security Protocols
- System Validation
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen