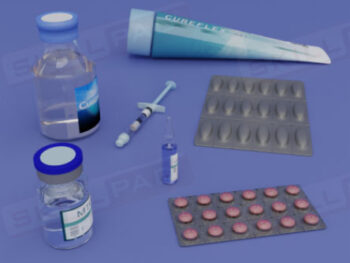
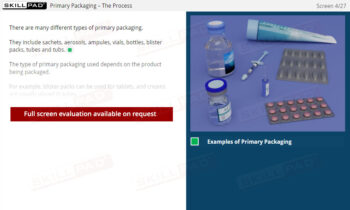
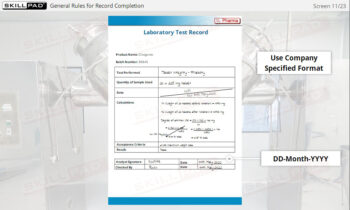
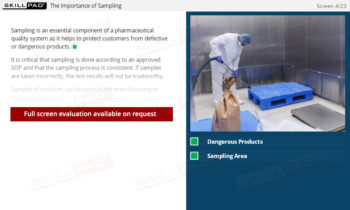
Secondary and Tertiary Packaging
An overview of the procedures and protocols involved in secondary and tertiary packaging in pharmaceutical manufacturing. This module covers the setup and operation of packaging equipment, the role of in-process checks in maintaining quality, the importance of reconciliation for tracking materials, and safety protocols for handling and clearing jammed materials. Learners will understand the significance of identification codes, batch records, and the final steps of tertiary packaging to ensure regulatory compliance and maintain product integrity during distribution.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
- Learn About Secondary and Tertiary Packaging: Understand key principles of secondary and tertiary packaging, including the roles of in-process checks, reconciliation, and identification codes.
- Recognize Common Packaging Issues: Learn about common challenges such as jammed materials and equipment malfunctions and gain practical knowledge to improve efficiency and problem-solving skills on the job.
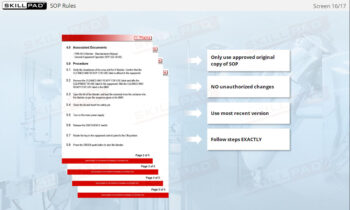
- Understand SOP Compliance: Learn the importance of adhering to SOPs for handling packaging materials, performing checks, and safely clearing jammed materials to support regulatory compliance and maintain safety.
Learning Objectives
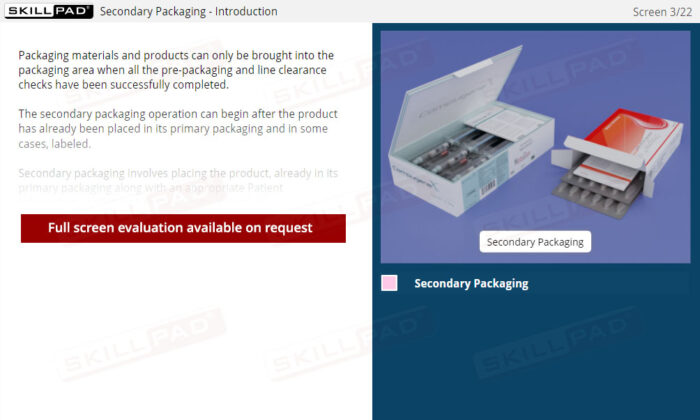
- Define the terms ‘secondary’ and ‘tertiary’ packaging.
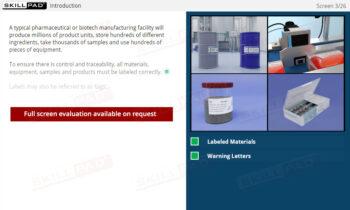
- Explain the purpose of identification codes in the secondary packaging process.
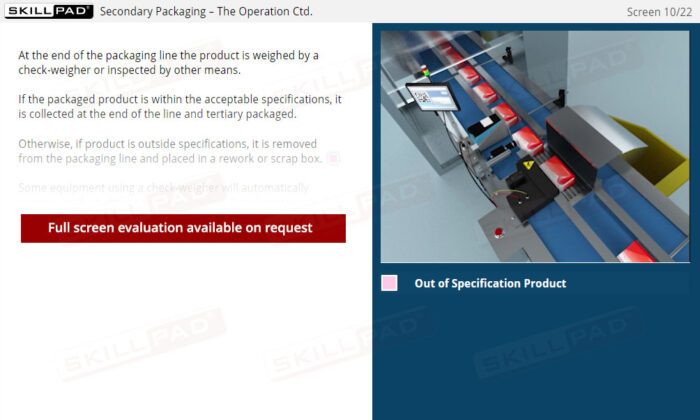
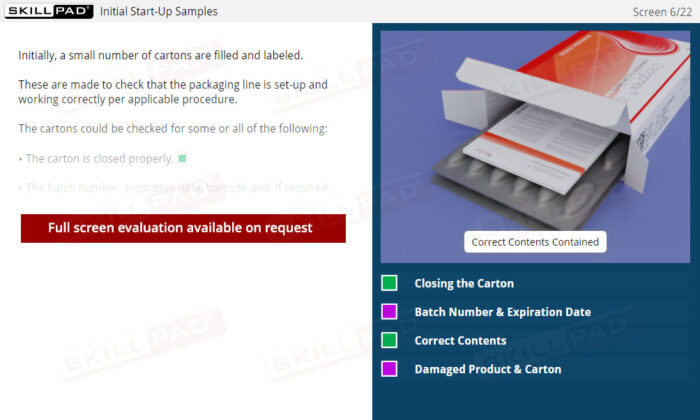
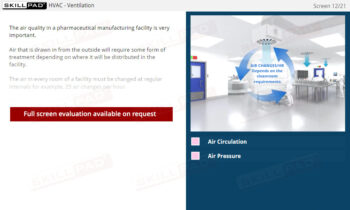
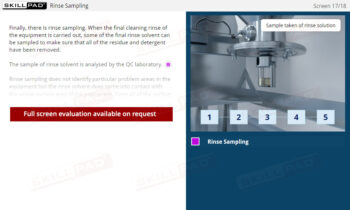
- List the in-process checks that are carried out during packaging operations.
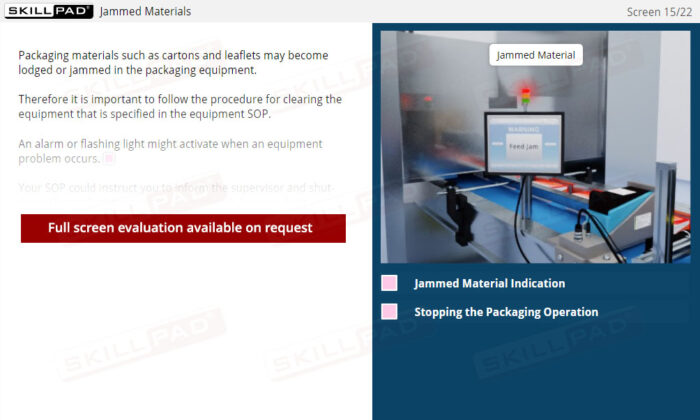
- Describe the removal process of jammed material from the packaging equipment.
- Define the term ‘reconciliation’.
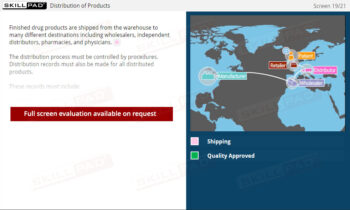
- Name the materials that have to be reconciled for secondary and tertiary packaging.
Keywords
- Batch Record
- Carton Inspection
- Check-Weigher
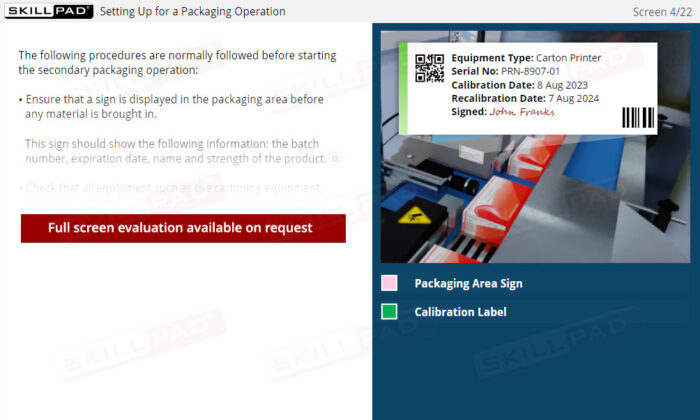
- Equipment Calibration
- GMP Compliance
- In-process Checks
- Jammed Material Removal
- Packaging Line
- Packaging Materials
- Packaging SOPs
- Patient Information Leaflet
- Pharmaceutical Packaging
- Reconciliation Process
- Secondary Packaging
- Tertiary Packaging
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen