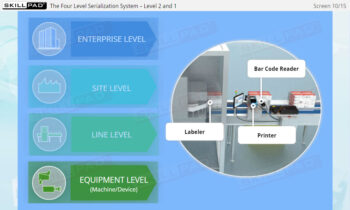
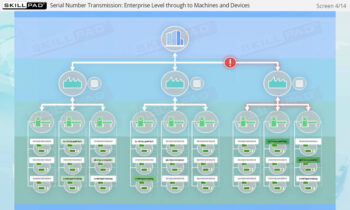
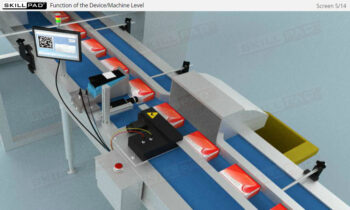
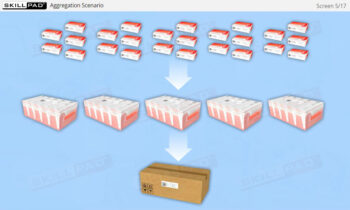
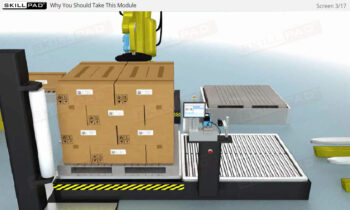
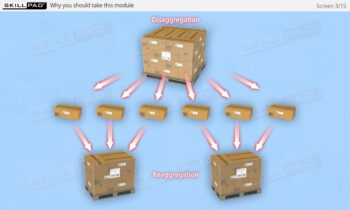
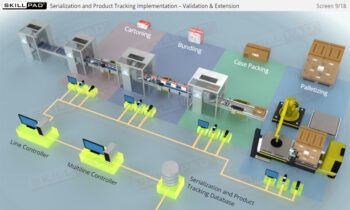
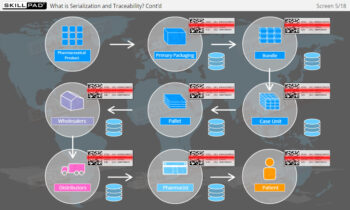
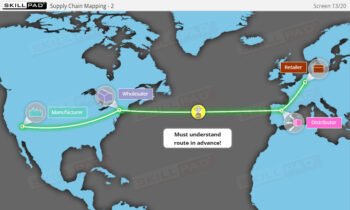
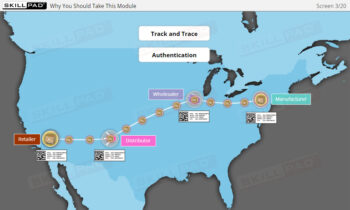
Founded in 1998 in Dublin, Ireland, Skillpad's team of industry-experienced Subject Matter Experts and Consultants believes Pharma, Biopharma, and Life Science Industry manufacturing companies can achieve production excellence with improved performance, productivity & compliance, as well as reduced losses & down-time, if they have KNOWLEDGE CONSISTENCY in all critical areas of manufacturing operations.
With 25 years in business, and offices in Europe, the USA and Canada, Skillpad has helped customers all over the world with Life Science Industry Knowledge challenges. We are fully dedicated to the idea that Knowledge Consistency is more achievable than ever using the latest technology and advanced knowledge transfer and management tools.