Finished Dose Contamination Prevention
An overview of how contamination can threaten the safety and effectiveness of finished dose drug products. This module explains the types and sources of contamination—microbiological, physical, and chemical—and emphasizes strategies to minimize risks through stringent hygiene, proper use of Personal Protective Equipment (PPE), and adherence to Good Manufacturing Practices (GMP). Learners will explore preventive measures and critical controls necessary to protect patients from potentially life-threatening contamination.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
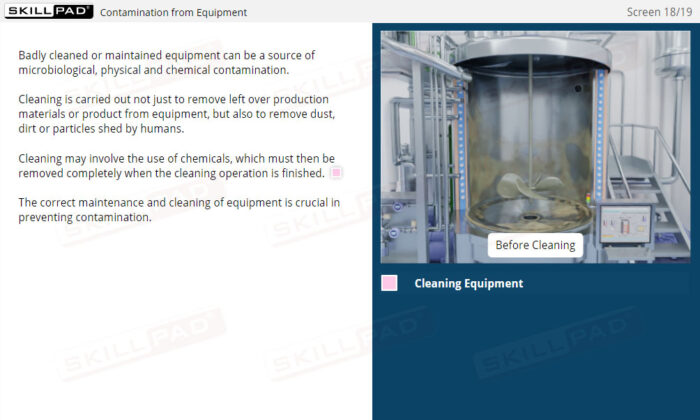
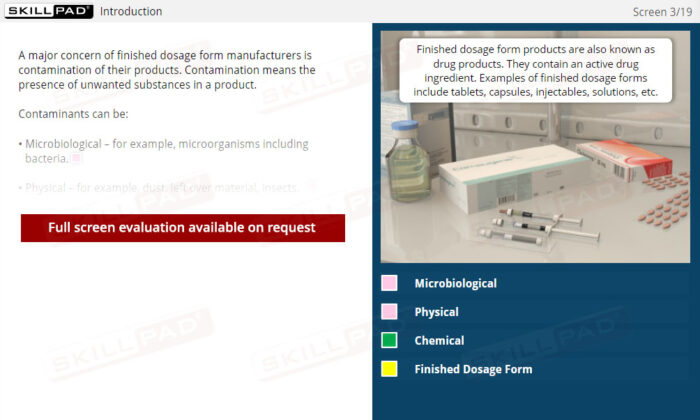
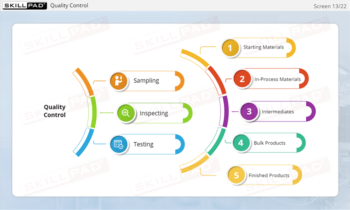
- Learn About Contaminants and Their Risks: Identify the different types of contaminants and understand their potential impact on drug product safety, enabling proactive contamination prevention measures.
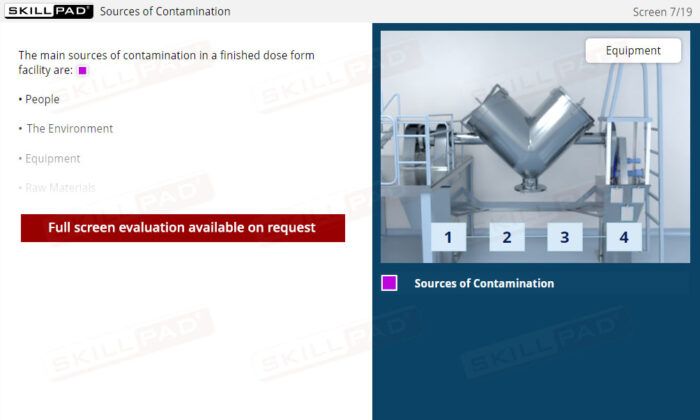
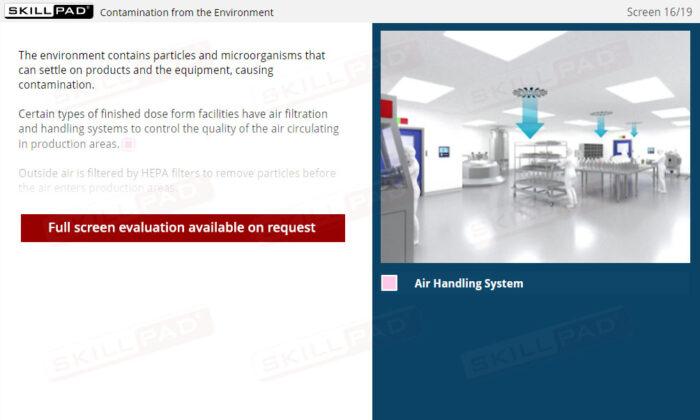
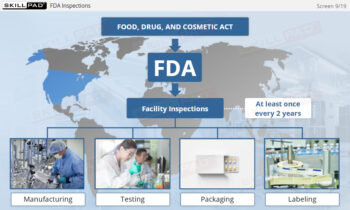
- Understand Sources of Contamination: Recognize how people, the environment, equipment, and raw materials can contribute to contamination, and learn practical methods to mitigate these risks within a manufacturing facility.
- Learn Contamination Prevention Techniques: Develop best practices for minimizing contamination risks, including effective use of PPE, adherence to hygiene protocols, and maintaining a clean production environment.
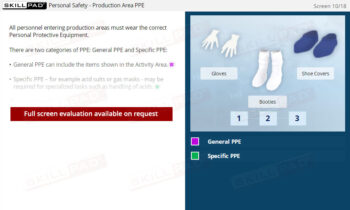
- Understand the Role of PPE: Learn the purpose of PPE and how to implement its correct use to protect both product integrity and employee health.
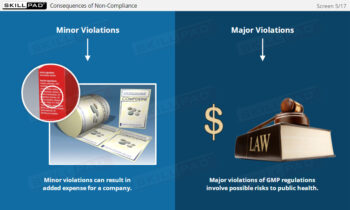
- Commit to a Hygiene and GMP Culture: Learn how consistent hygiene habits and adherence to GMP are essential for ensuring product safety, quality, and regulatory compliance.
Learning Objectives
- List the different types of contaminants that can be found in a finished dose manufacturing facility.
- Identify the main sources of contamination in a finished dose manufacturing facility.
- Describe how to minimize the risk of product contamination.
- Describe Personal Protective Equipment (PPE) and its purpose.
Keywords
- Air Filtration
- Chemical Contamination
- Contamination Prevention
- Finished Dose Manufacturing
- Good Manufacturing Practices (GMP)
- Hygiene Practices
- HVAC Systems
- Microbiological Contamination
- Personal Protective Equipment (PPE)
- Physical Contamination
- Raw Material
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen