Overview of Pharmaceutical Manufacturing
An overview of the foundational principles of pharmaceutical manufacturing, covering the different types of pharmaceutical products and the distinction between Active Pharmaceutical Ingredients (APIs) and finished dose products. This module provides insights into the complex processes involved in bringing a new drug to market, including drug discovery, clinical trials, regulatory approval, and post-market surveillance. Learners will also become familiar with the key departments within a pharmaceutical manufacturing facility – such as Production, Quality, Logistics, Engineering, and IT – and understand their essential roles in ensuring the safety, efficacy, and quality of drug products.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Core Library
Description
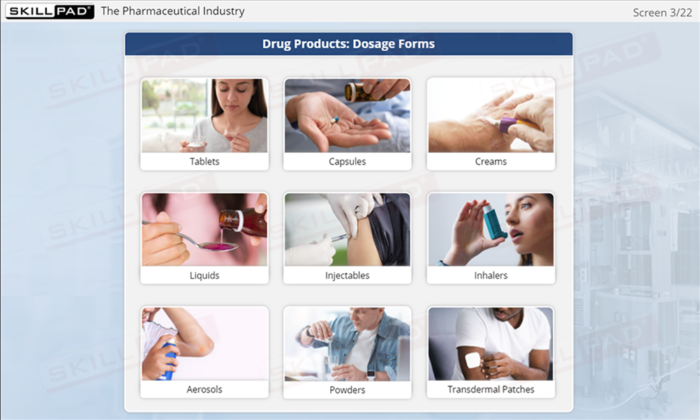
- Explore Pharmaceutical Product Types: Identify and differentiate between various pharmaceutical dosage forms, such as tablets, aerosols, injectables, and more.
- Distinguish Between APIs and Finished Dose Products: Learn the roles of Active Pharmaceutical Ingredients and excipients in drug formulation to build a foundational understanding of manufacturing processes.
- Gain Insights into Drug Development and Regulatory Approval: Gain insights into the drug development process, from initial drug discovery to post-marketing surveillance, and understand the significance of each stage.
- Gain Functional Knowledge of Key Departments: Become familiar with the roles of departments such as Production, Quality, and Logistics, and how they collaborate to uphold GMP standards and ensure timely product distribution.
- Understand Quality and Compliance: Learn about the importance of Good Manufacturing Practice (GMP) and the responsibilities employees have in maintaining product safety, efficacy, and purity.
- Prepare for Real-World Applications: Gain foundational knowledge needed for roles in pharmaceutical manufacturing, improving your readiness to contribute effectively in a regulated, multidisciplinary environment.
Learning Objectives
- List examples of different types of pharmaceutical products.
- Explain the difference between an API and a finished dose product.
- Explain the term ‘excipient’.
- Describe the different stages involved in bringing a new pharmaceutical product to market.
- List the different departments typically found in a pharmaceutical manufacturing facility.
- Describe the functions of the different departments typically found in a pharmaceutical manufacturing facility.
Keywords
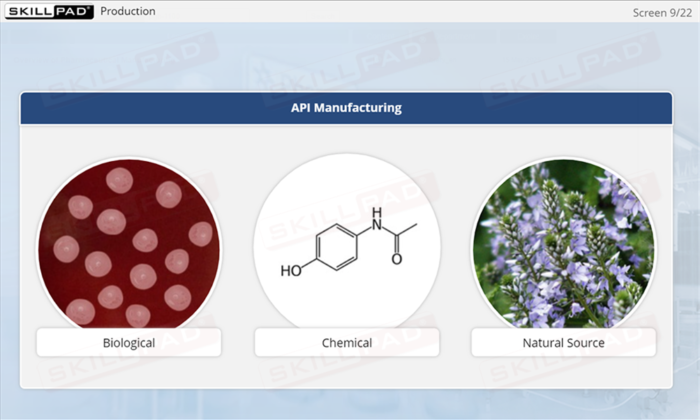
- Active Pharmaceutical Ingredient (API)
- Clinical Trials
- Drug Discovery
- Excipients
- Finished Dose Product
- Good Manufacturing Practice (GMP)
- Logistics Department
- Pharmaceutical Manufacturing
- Post-Marketing Surveillance
- Pre-Clinical Studies
- Production Department
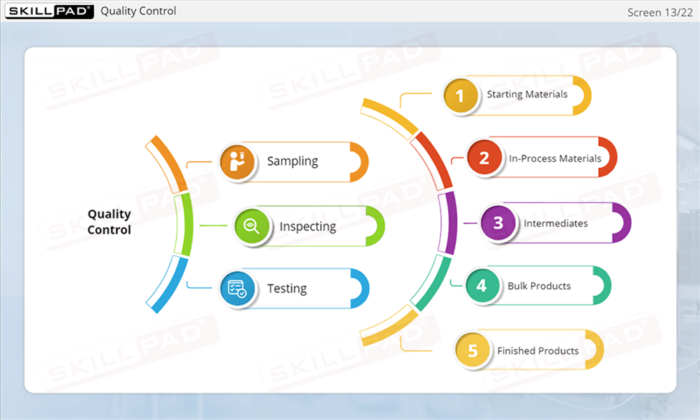
- Quality Control
- Regulatory Approval
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen