GCP Inspection Readiness – Close
Explore the critical tasks and best practices for concluding a GCP inspection readiness project. This module guides learners through the Close phase, including how to conduct a close-out meeting, respond to inspector observations, and prepare an internal report to document lessons learned.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
- Gain Insight into Effective Inspection Closure: Learn how to successfully navigate the final steps of a GCP inspection readiness project, ensuring that all observations are addressed, and that the inspection is closed out effectively.
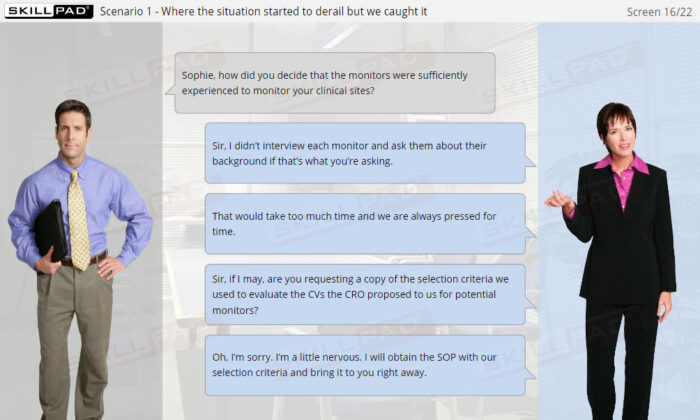
- Respond to Inspector Observations with Accuracy: Develop a clear approach for handling and addressing observations in a close-out meeting, ensuring timely and compliant responses.
- Improve Documentation and Reporting: Recognize the importance of preparing comprehensive internal reports, capturing key insights, and implementing corrective actions to strengthen future compliance efforts.
- Enhance Team Collaboration and Debriefing: Learn how to conduct post-inspection debriefs and internal meetings to consolidate feedback and drive continuous improvement.
- Apply Project Management Strategies to Inspection Closure: Engage in practical applications of project management techniques to support accurate and efficient completion of final inspection tasks.
Learning Objectives
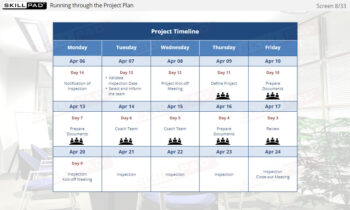
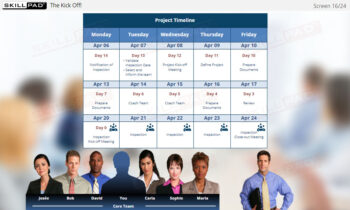
- Recall the key points from the third phase of an Inspection Readiness Project Plan – ‘Execute and Monitor’.
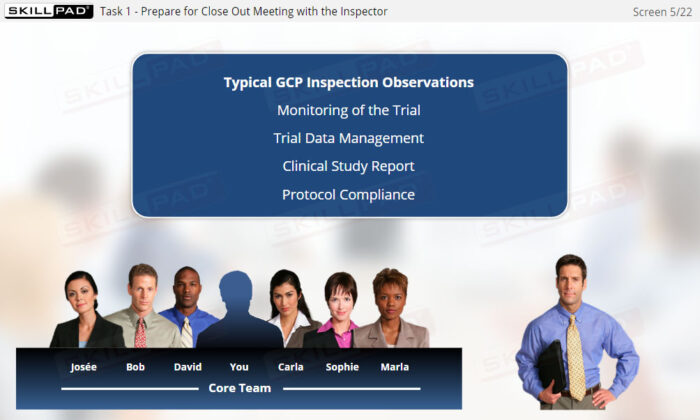
- List areas where observations are typically made in a GCP Inspection.
- Describe the purpose of holding a close out meeting with an inspector.
- Explain why an internal team meeting should be held before the close out meeting.
- List the key tasks involved in the Close phase of an inspection readiness project.
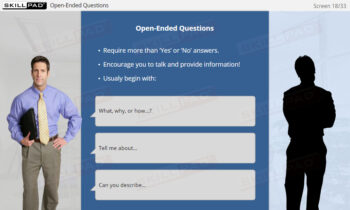
- Explain the importance of responding correctly to inspection observations.
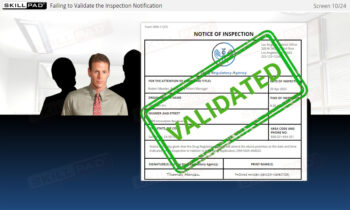
- Describe the consequences that can result from not executing or failing to execute key tasks in the Close phase of an inspection.
- Explain the value of preparing an internal inspection report.
- Apply project management principles and techniques to the Close phase of an inspection readiness project.
Keywords
- Close Phase
- GCP Inspection Readiness
- Inspection Preparation
- Internal Reporting
- Project Management
- Observation Response
- Regulatory Compliance
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen