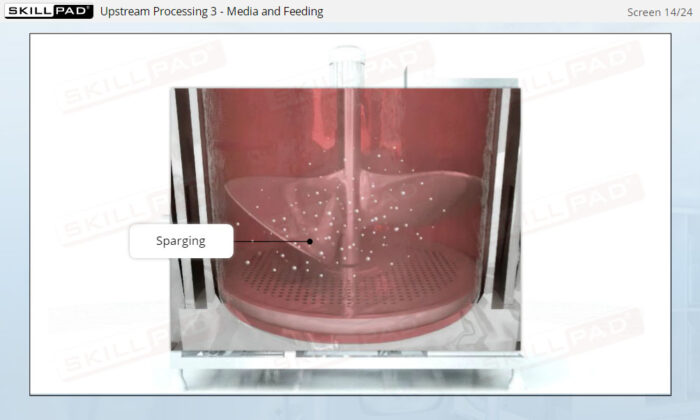
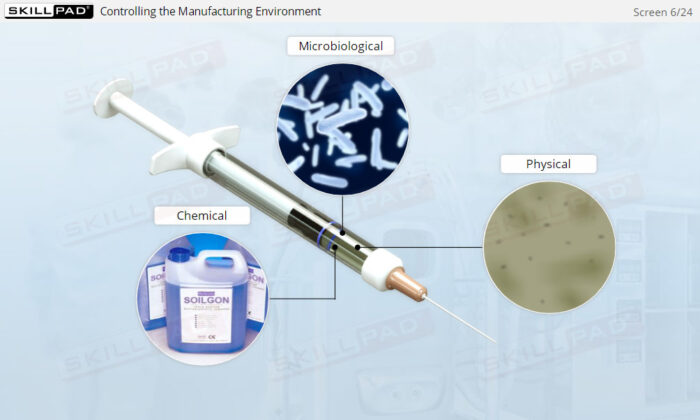
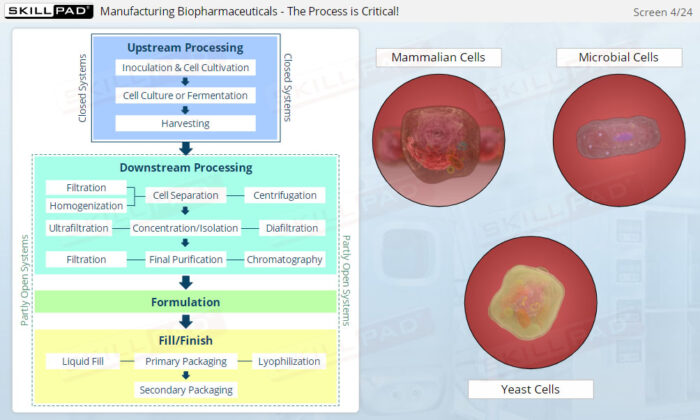
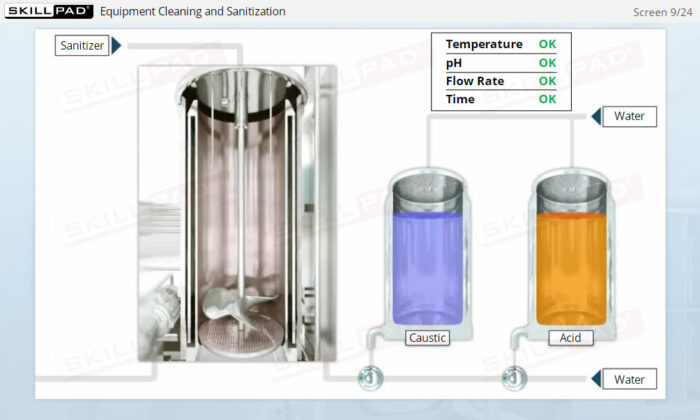
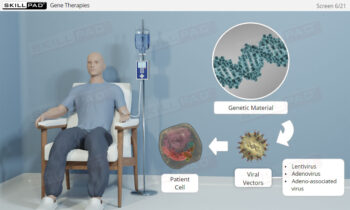
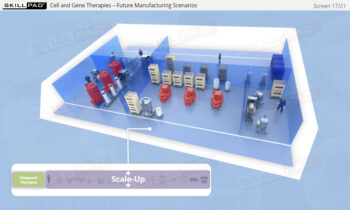
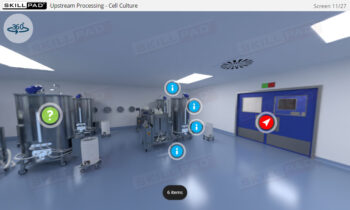
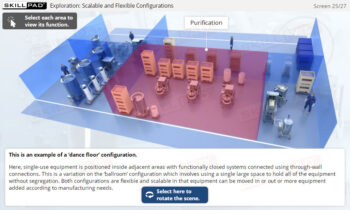
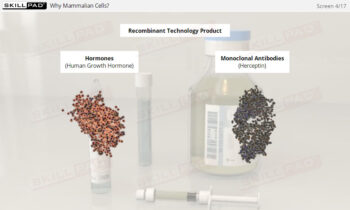
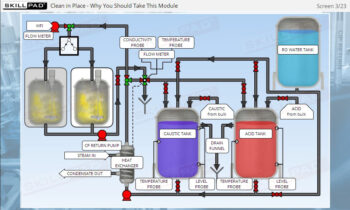
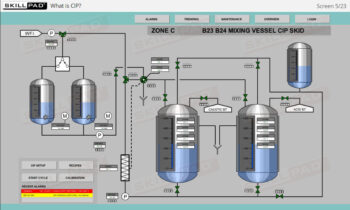
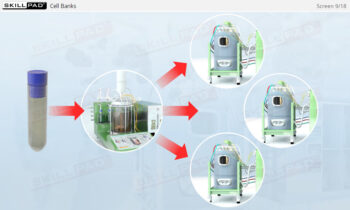
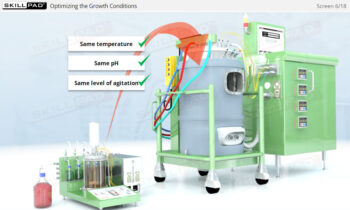
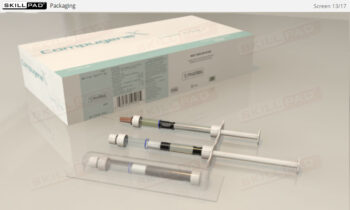
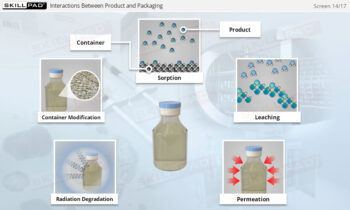
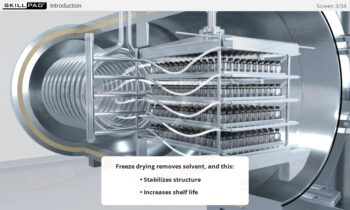
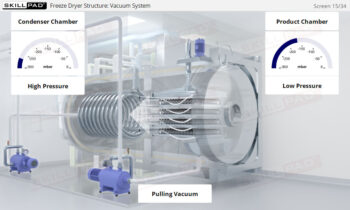
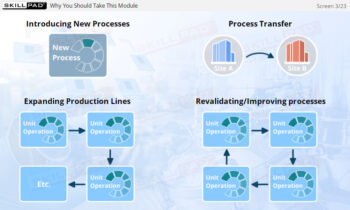
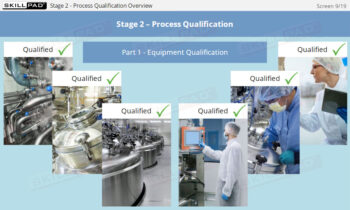
Overview of Biopharmaceutical Manufacturing
This Module is aimed at biopharmaceutical manufacturing personnel who require an overview of the main processes and conditions involved in therapeutic protein manufacture including upstream processing, downstream processing as well as formulation and fill finish.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Description
Learning Objectives:
Learner Benefits:
|
Module Features:
Animations |
|---|