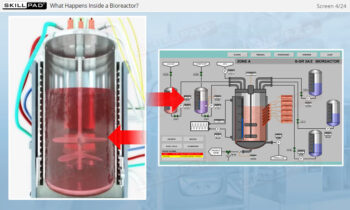
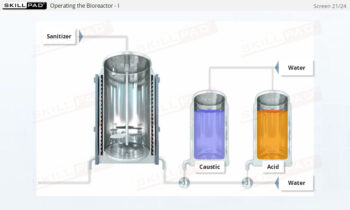
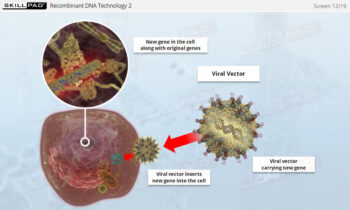
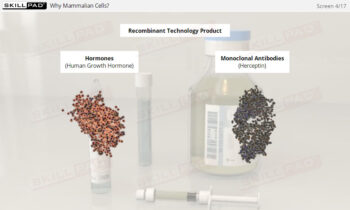
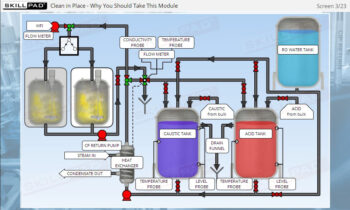
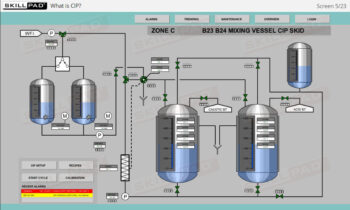
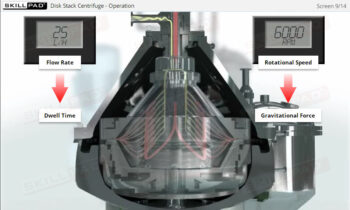
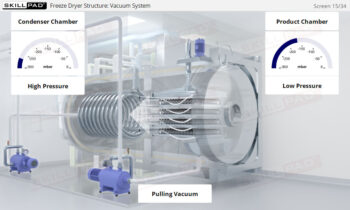
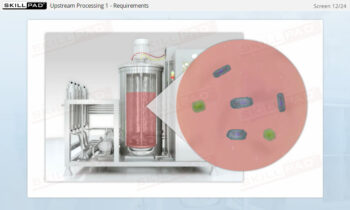
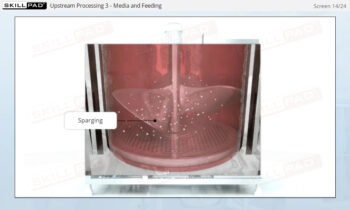
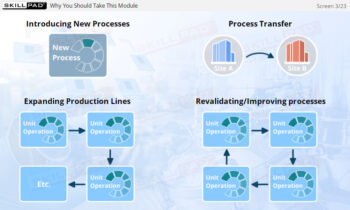
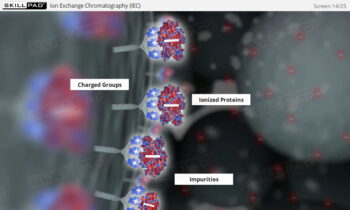
Process Validation: Process Qualification and Control
An overview of the qualification and continuing verification stages of process validation, intended to demonstrate that a biopharmaceutical process is capable of reproducible commercial manufacturing and to provide ongoing assurance that the process remains in a state of control.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Grade: Premium Performance
Description
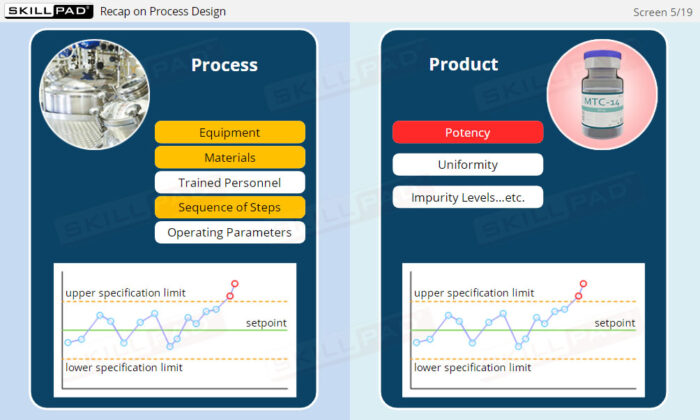
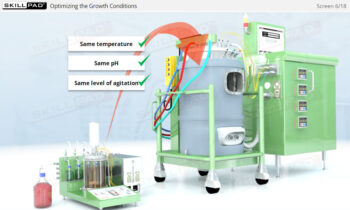
- Gain a comprehensive understanding of the critical stages of process validation—Process Qualification and Continued Process Verification-and their roles in achieving consistent product quality.
- Learn how to confirm that manufacturing processes are capable of reproducible, commercial-scale production through a detailed exploration of qualification and control strategies.
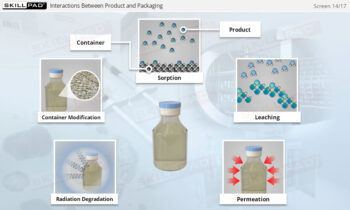
- Acquire knowledge of essential assessment tools and techniques, including statistical analysis and sampling plans, to verify process consistency and quality.
- Understand the role of change control in assessing process changes and how to implement requalification to maintain compliance and product integrity.
- Recognize the importance of cross-functional teamwork, involving production, quality, engineering, and validation teams, to maintain process control and enhance product quality.
Learning Objectives
- Explain the purpose of process qualification.
- State the pre-requisites that must be in place before process qualification can proceed.
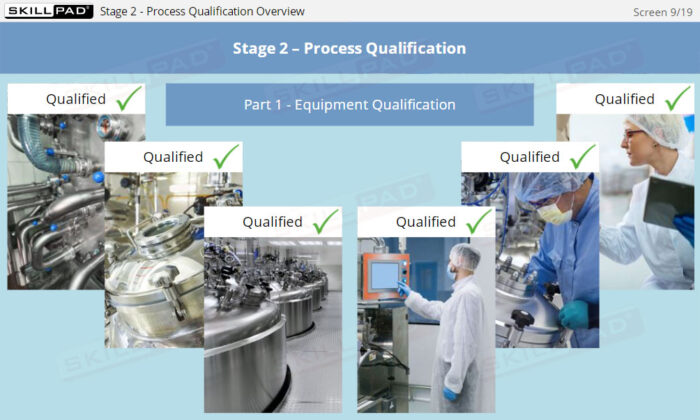
- Summarize the activities that occur in process qualification.
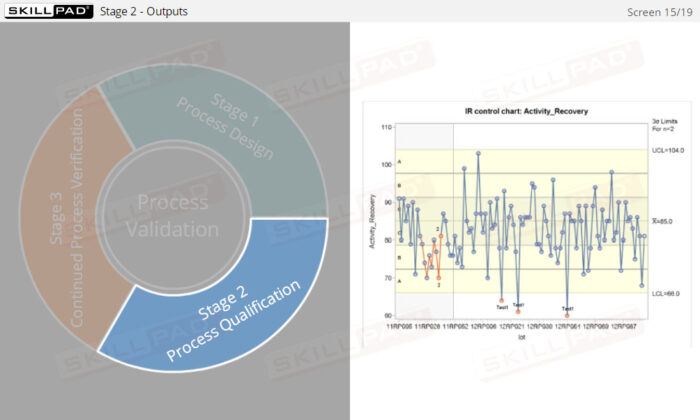
- Explain the purpose of continued process verification.
- Describe some of the assessment tools used in continued process verification.
- Explain the role of change control in assessing changes to validated processes.
- Provide an example of where requalification would be necessary.
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen