Aseptic Processing: Contamination Control
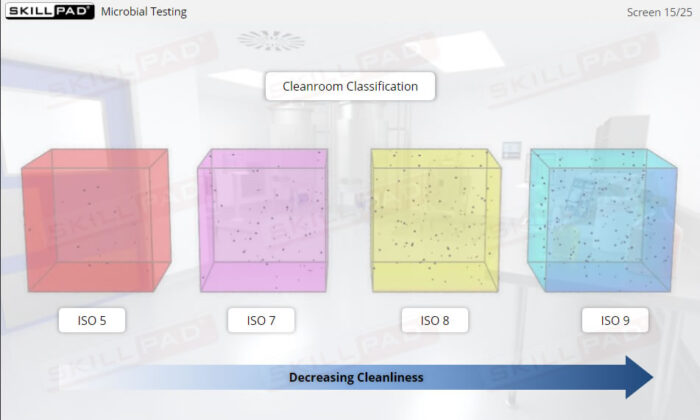
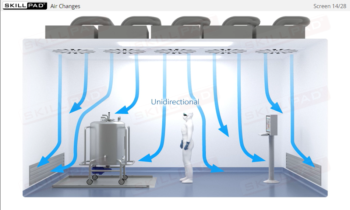
Contamination prevention measures, procedures, and practices that are typically implemented in aseptic processing including the use of cleanrooms, cleanroom gowning, cleanroom cleaning procedures, microbial testing, correct cleanroom behavior, and appropriate personal hygiene.
Part of Annex 1 training requirements.
Whether for onboarding or annual refresher training, this Module is seamlessly deployable on any LMS and can be tailored to your company’s exact needs.
Duration: 30 Mins
Description
Learning Objectives:
Learner Benefits:
|
Module Features:
Animations |
|---|
Keywords
- Aseptic Processing
- Cleanrooms
- Cleanroom Behavior
- Cleaning Procedures
- Contamination Control
- Gowning, Microbiology
- Microbial Testing
- Personal Hygiene
- Regulatory Compliance
- Quality Assurance