Aseptic Processing: Essential Personnel Practices
New Module – Now Available!
Personnel are the primary source of contamination in aseptic manufacturing, which means their movements, behavior, and gowning practices have a direct impact on product integrity and patient safety.
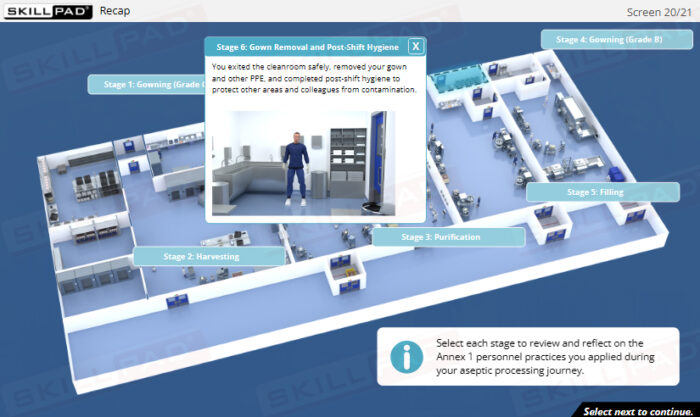
Skillpad’s Essential Personnel Practices e-Lesson places learners inside an interactive aseptic processing environment where they follow the manufacturing journey of a fictional product. Through immersive 3D scenes and practical, task-based activities, users learn the how and why of contamination prevention across gowning and degowning, aseptic techniques, cleanroom behavior, and hygiene and health compliance, all aligned with EU GMP Annex 1 requirements.
Description
- Regulatory Compliance with EU Annex 1: Understand key personnel-related requirements for aseptic processing and how to align daily cleanroom practices with regulatory expectations.
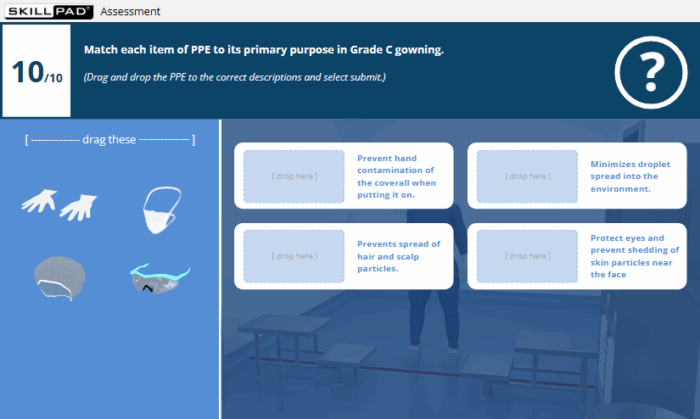
- PPE Purpose and Gowning Discipline: Learn the purpose of critical PPE items and apply the correct behaviours, hygiene practices, and dos and don’ts for working while gowned in Grade C and Grade B cleanrooms.
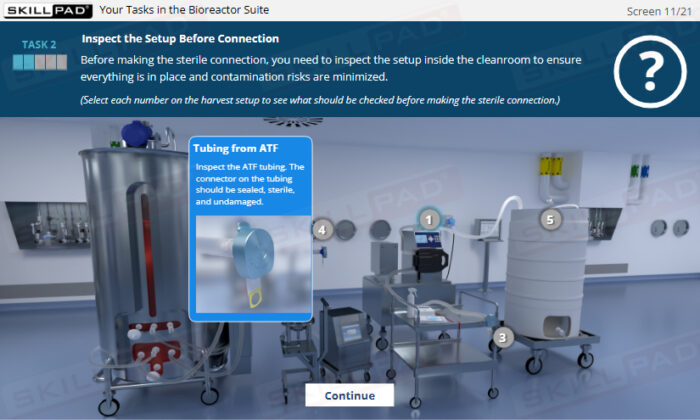
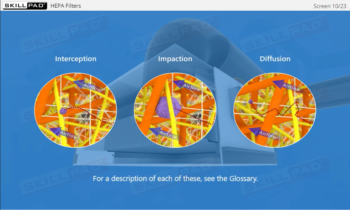
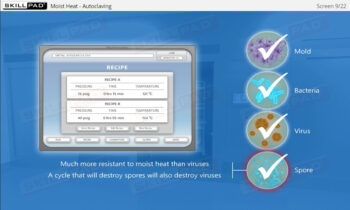
- Enhanced Awareness of Contamination Control: Recognize how personnel behaviours, movement, and hygiene influence contamination risks and how to protect product sterility.
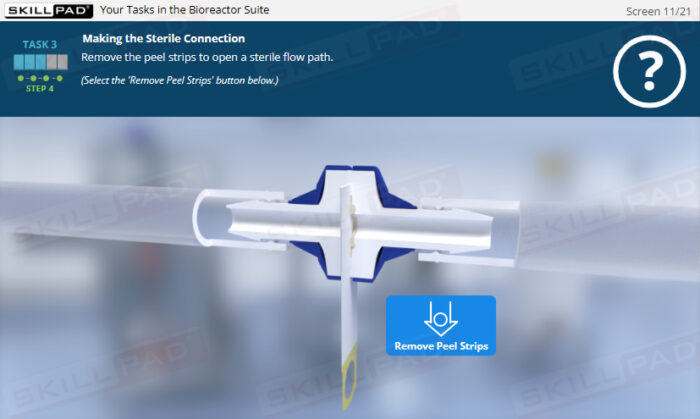
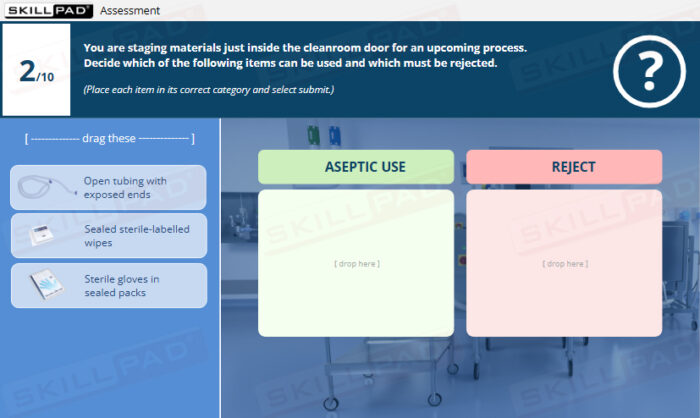
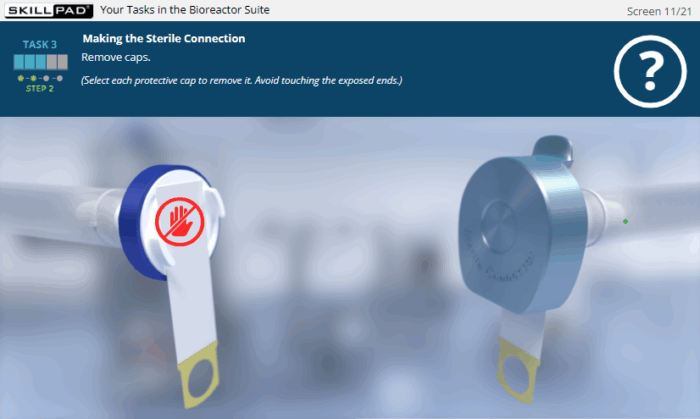
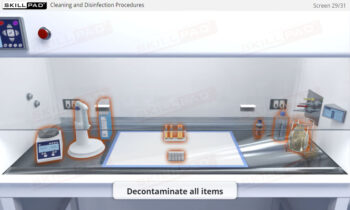
- Application of Best Practices in Aseptic Environments: Engage in interactive scenarios that reinforce correct cleanroom conduct, aseptic handling, sterile-connection technique, and contamination-prevention strategies.
- Confidence in Addressing Non-Compliance: Develop the ability to identify, respond to, and escalate personnel-related deviations that could compromise aseptic integrity.
Learning Objectives
- Identify key EU Annex 1 regulatory requirements for personnel working in aseptic processing of sterile medicinal products.
- Describe the correct gowning procedures for different cleanroom grades to minimize contamination risks.
- Recognize critical aseptic behaviors that support contamination control and protect product sterility in cleanrooms.
- Explain essential health and hygiene standards required to prevent contamination risks in aseptic environments.
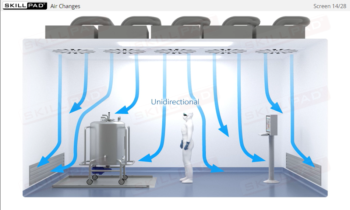
- Apply best practices for personnel movement and conduct to reduce particle shedding and maintain controlled conditions.
- Demonstrate an understanding of common personnel-related non-compliances and the corrective actions needed to uphold aseptic integrity.
Keywords
- Aseptic Processing
- Cleanroom Behavior
- Gowning Procedures
- Contamination Prevention
- EU Annex 1 Compliance
- Personnel Practices
- Sterile Manufacturing
- Aseptic Technique
Module Features
Animations
Voice Over
Knowledge Checks
Assessments
SCORM/AICC compatible
Full Screen